Bücher und Sonderbände

13.) M. H. G. PRECHTL*, S. Sahler, " Ionic Liquids for Hydrogen Storage: Opportunities for Organometallic Chemistry" in “Advances in Organometallic Chemistry and Catalysis: The Silver/Gold Jubilee International Conference on Organometallic Chemistry Celebratory Book”, 2014, ch. 40, p. xxx-XXX, edited by A. Pombeiro, Wiley-Blackwell, Weinheim, ISBN 9781118510148. DOI: 10.1002/9781118742952.ch40
Keywords:
ionic liquids, hydrogen storage; catalysis; metal complexes; ammonia borane; formic acid
Summary
Small molecules such as formic acids as well as ammonia borane and its derivatives are highly interesting for hydrogen storage. These compounds release hydrogen gas in presence of organometallic complexes as well as metal catalysts derived from such precursors at comparably low temperatures. The efficiency of such catalytic systems can be further improved by application of ionic liquids (IL) regarding: (I) stabilization of the catalyst, (II) recycling of the catalyst, (III) promoted dehydrogenation in case of ammonia borane derivatives and (IV) solubilization of hardly soluble dehydrogenated “spent-fuel”- products derived from ammonia borane derivatives. All these factors imply that additional material (e. g., IL) can still be beneficial for overall efficiency of a hydrogen storage system. Moreover, the immobilization of metallic catalysts in IL also gives access to a recyclable catalyst systems regarding the challenging regeneration of the spent-fuels (CO2 and dehydrogenated amino-borane products). So far, there are only very few examples applying organometallic catalyst (precursors) in ionic liquid media for dehydrogenation and hydrogenation reactions for potential future hydrogen storage materials.
________________________________________________________________
12.) M. H. G. Prechtl*, P. S. Campbell, Nanotechnol. Rev.. 2013, DOI: 10.1515/ntrev-2013-0019
. "Metal Oxide and Bimetallic Nanoparticles in Ionic Liquids: Synthesis and Application in Multiphase Catalysis"
ABSTRACT:
Ionic liquids (ILs) are well established as solvents and stabilising agents for the synthesis of metallic nanoparticles (NPs) in general. The physico-chemical properties of ILs and the supramolecular organisation in the liquid state, are capable of directing the growth of transition metal NPs generated in situ, and to subsequently protect and stabilise them. Until now, many different NPs have been successfully synthesised within these media, however the synthesis of metal oxide and bimetallic alloy or core-shell nanoparticles in ionic liquids is still relatively rare. Herein we summarise the current state of the art of the synthetic methods for these materials and their application in the broad field of catalysis including multiphase systems, hydrogenation, dehydrogenation, functionalisation as well as defunctionalisation reactions.
________________________________________________________________
11.) P. S. Campbell*, M. H. G. Prechtl*, C. C. Santini*, P. H. Haumesser, Curr. Org. Chem. 2013, 17, 4, 414-429. " Ruthenium Nanoparticles in Ionic Liquids – A Saga" (Special Issue: "Nanoscale Catalysts as Tools for Synthesis" - guest editor: M. H. G. Prechtl) DOI: 10.2174/1385272811317040008
SPECIAL ISSUE: Nanoscale Catalysts as Tools for Synthesis
Abstract:
Ionic liquids (ILs) are excellent media for the generation and stabilisation of metallic nanoparticles (NPs). Their ionic character coupled with 3-D structural pre-organisation in the liquid state, serves to direct the growth of transition metal NPs generated in situ, and to subsequently protect and stabilise them. Until now, many different NPs have been successfully synthesised within these media, however much attention has been paid to Ru-NPs. These have been prepared with small sizes and narrow size distributions by reduction of organometallic compounds with molecular hydrogen as well as decomposition of transition-metal complexes in the zero-valent state. These stable Ru-NPs immobilised in the ILs have proven to be efficient green catalysts for several reactions in multiphase conditions, including important energy-related processes such as biomass refinement. Furthermore, they present potential novel materials for use in the production of smarter electronic devices. In this review, the synthesis, stabilisation and size-control of Ru-NPs via various methods in different ILs is discussed, followed by their varied application in catalysis and potential in new fields.
________________________________________________________________
10.) M. H. G. Prechtl*, S. Sahler, Curr. Org. Chem. 2013, 17, 3, 220-228. "Hydrogen Storage Using Ionic Liquid Media" (Special Issue: "Between Revolution and Ignorance: The Two Worlds of Ionic Liquids" - guest editor: B. A. D. Neto) DOI: 10.2174/1385272811317030004
In the field of hydrogen storage, ionic liquids (ILs) find application as: solvent, co-catalyst, catalyst, hydrogen storage material and stabilizing agent. Benefits of ILs for the system are discussed here.
________________________________________________________________
9.) S. Sahler, M. H. G. PRECHTL* "Application of Ionic Liquids for Hydrogen Storage Systems" in “Hydrogen Storage” edited by J. Liu, InTech, Vienna, (personal invitation), ISBN 980-953-307-354-0. DOI: 10.5772/50154
________________________________________________________________
8.) M. T. Keßler, J. D. Scholten, F. Galbrecht, M. H. G. PRECHTL* "Coupling Reactions in Ionic Liquids" in “Palladium-Catalyzed Cross-Coupling Reactions - Practical Aspects and Future Developments”, 2013, ch. 6, p. 201-231, edited by A. Molnar, Wiley-VCH, Weinheim, ISBN 978-3-527-33254-0. DOI: 10.1002/9783527648283.ch6
________________________________________________________________
7.) , M. T. Keßler, J. D. Scholten, M. H. G. PRECHTL* "Metal Catalysts immobilised in Ionic Liquids: A Couple with Opportunities for Fine Chemicals Derived from Biomass" in “Catalytic Hybrid Materials, Composites, and Organocatalysts”, 2013, ch. 10, 243-264 (Book series: New and the Future Developments in Catalysis”) edited by S. Suib, Elsevier, Amsterdam, ISBN 978-0-444-53876-5. DOI: 10.1016/B978-0-444-53876-5.00010-6
________________________________________________________________
6.) J. D. Scholten, M. H. G. PRECHTL, J. Dupont, “Formation of Nanoparticles Assisted by Ionic Liquids” in “Handbook of Green Chemistry - Green Processes (Vol. 8 - Green Nanoscience)” edited by P. T. Anastas (series editor), WILEY-VCH, Weinheim, April 2012. ISBN-10: 3-527-31576-4
ISBN-13: 978-3-527-31576-5
Online ISBN: 9783527628698
http://dx.doi.org/10.1002/9783527628698.hgc085
Abstract:
The sections in this article are
1.1 Metal Nanoparticles in Ionic Liquids: Synthesis
1.1.1 Reduction of Organometallic Precursors
1.1.2 Decomposition of Organometallic Precursors
1.1.3 Transfer from an Aqueous/Organic Phase to the Ionic Liquid Phase
1.1.4 Bombardment of Bulk Materials
1.2 Metal Nanoparticles in Ionic Liquids: Stabilization
1.3 Metal Nanoparticles in Ionic Liquids: Recyclable Multiphase Catalyst-Systems
1.3.1 Hydrogenation of Multiple Bonds with Metal Nanoparticles in Ionic Liquids
1.3.2 Carbon–Carbon Cross-Coupling Reactions Catalyzed by Palladium Nanoparticles in Ionic Liquids
1.3.3 Functionalization and Defunctionalization Reactions Using Metal Nanoparticles in Ionic Liquids
1.3.4 Isotope Exchange Catalyzed by Metal Nanoparticles in Ionic Liquids
1.3.5 Application of Metal Nanoparticle Catalysts in Ionic Liquids for Energy- and Environment-Related Systems
1.4 General Remarks
Keywords: nanoparticles; ionic liquids; catalysis; recyclability; green chemistry
________________________________________________________________
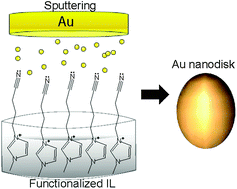
5.) H. Wender, P. Migowski, A. F. Feil, L. F. de Oliveira, M. H. G. Prechtl, R. Leal, G. Machado, S. R. Teixeira, J. Dupont, Phys. Chem. Chem. Phys. 2011, 13, 13552-13557. "On the Formation of Anisotropic Gold Nanoparticles by Sputtering onto a Nitrile Functionalised Ionic Liquid". http://dx.doi.org/10.1039/c1cp21406c (Special issue on the theme of "Nanostructures in Ionic Liquids")
Abstract:
Sputtering deposition of gold onto the 1-(butyronitrile)-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (BCN)MI·N(Tf)2 ionic liquid (IL) has generated colloidal and stable gold nanospheres (AuNS) and gold nanodisks (AuND) in a bimodal size distribution. Upon increasing the sputtering discharge voltage, three distinct phenomena were observed: (i) the mean diameter of both AuNS and AuND decreased; (ii) the population with lower diameters increased and (iii) the formation of AuND disappeared at voltages higher than 340 V. By dissolving the colloidal gold nanoparticles (AuNPs) in isopropanol and dropping the product onto carbon-coated Cu grids, 2D and 3D superlattices tended to be formed, as observed by transmission electron microscopy (TEM). Therefore, the formation of AuND is probably related to a strong interaction between sputtered Au atoms of low kinetic energy and the nitrile groups orientated to the vacuum phase of the IL surface, which drives the preferential anisotropic lateral growth.
________________________________________________________________
4.) M. H. G. PRECHTL*, J. D. Scholten, J. Dupont, „Palladium Nanoscale Catalysts in Ionic Liquids: Coupling and Hydrogenation Reactions” in “Ionic Liquids, Theory and Applications” (Editor: Alexander Kokorin), INTECH, Vienna (Austria), 2011, 393-414. ISBN 978-953-307-248-7 (personal invitation) http://www.intechopen.com/articles/show/title/palladium-nanoscale-catalysts-in-ionic-liquids-coupling-and-hydrogenation-reactions
Zusammenfassung (Englisch):
The promising potential of nanoscale catalysts and ionic liquids is reflected in numerous publications in the last two decades. Sustainable and recyclable synthetic methods are requested nowadays; therefore novel environmental benign catalyst systems have been under development in the recent years. Ionic liquids revealed great potential as catalyst-stabilising solvent for multiphase reaction systems and as medium for the synthesis of metal nanoparticles in general. Such metal nanoparticles show interesting properties for heterogeneous as well as for homogeneous catalytic reactions in organic chemistry. Their advantage combines the long catalyst life-time of solid catalyst with the high activity of molecular complex catalysts.
In the present chapter a selection of palladium catalysed coupling and hydrogenation reactions in ionic liquids (ILs) is presented. Model systems using the Pd-NPs/ILs approach are presented for homogeneous and heterogeneous reactions: Cross-coupling reactions like the Heck and Suzuki reaction, for example, are discussed besides others. In such reactions the palladium nanoparticles act as reservoir for the catalytically active palladium species in IL-based multiphase system. Complete and partial hydrogenation reactions of double and triple bonds are presented. The sub-chapters include perspectives about catalyst recyclability, reaction pathways and mechanistic aspects.
________________________________________________________________
3 .) M. H. G. Prechtl*, J. D. Scholten, J Dupont, "Carbon-Carbon Cross Coupling Reactions in Ionic Liquids Catalysed by Palladium Metal Nanoparticles", Molecules 2010, 15, 3441-3461. http://dx.doi.org/10.3390/molecules15053441 (Special Issue: "Nano-catalysts and Nanotechnologies for Green Organic Synthesis")
Abstract:
A brief summary of selected pioneering and mechanistic contributions in the field of carbon-carbon cross-coupling reactions with palladium nanoparticles (Pd-NPs) in ionic liquids (ILs) is presented. Five exemplary model systems using the Pd-NPs/ILs approach are presented: Heck, Suzuki, Stille, Sonogashira and Ullmann reactions which all have in common the use of ionic liquids as reaction media and the use of palladium nanoparticles as reservoir for the catalytically active palladium species.
Keywords: palladium; nanoparticles; ionic liquids; cross-coupling; Heck; Suzuki; Stille; Sonogashira; Ullmann
________________________________________________________________
2.) M. H. G Prechtl*, J. D. Scholten, B. A. D. Neto, J. Dupont, Curr. Org. Chem. 2009, 13, 1259-1277. "Application of Chiral Ionic Liquids for Asymmetric Induction in Catalysis", (personal invitation). http://dx.doi.org/10.2174/138527209789055153
Abstract:
Here we present the state-of-the-art for asymmetric catalysis using chiral ionic liquids (CILs) as source of chiral information. The current review covers reactions using typical homogeneous catalysts e.g. organocatalysts, transition metal complexes and solid catalysts for heterogeneous catalysis in solvent-systems with chiral ionic liquids.
________________________________________________________________
1.) M. H. G. PRECHTL, „Novel Ruthenium Dihydrogen Complexes and their Application in Catalysis.“ WiKu-Verlag (Publisher) Series – Stone´s Publishing Cologne, Germany. 2007, 167 pages (book), Vol 16.: ISBN-13: 978-3-86553-229-9, ISBN-10: 3865532292. PhD-thesis, RWTH Aachen 2007, Aachen. http://darwin.bth.rwth-aachen.de/opus3/volltexte/2007/1991/ http://www.stonespublishingcologne.eu/12650.html
Zusammenfassung:
Die Synthese der nicht-klassischen Rutheniumhydridkomplexe [Ru(dtbpmp)H2(H2)] (PNP Pincer-Rückgrat) und [Ru(dtbpoet)(H2)H2] (POP- Pincer-Rückgrat) mit fixierter Geometrie, die Anwendung in homogener Katalyse und ihre Reaktivität gegenüber kleinen Molekülen wie N2, CO2 und CO wird beschrieben. Die Komplexe mit “side-on”-gebundenen molekularen Wasserstoff wurde analysiert mittels NMR (T1-analysis), FT-IR und DFT-Rechnungen. Nichtklassischen Rutheniumhydridkomplexe haben ein grosses Potential als Katalysatoren für CHAktivierung, speziell für den H/D-Austausch und Wasserstofftransferprozesse. Wir haben erfolgreich einen neuen Rutheniumhydridkatalysator für den H/D-Austausch zwischen (hetero)aromatischen oder olefinischen Protonen und D2O (oder C6D6) unter milden Bedingungen eingesetzt. Der H/D-Austausch umfasst auch mechanistische Untersuchungen (NMR-Kinetik und DFT-Rechnungen). Interessanterweise ist der Rutheniumkomplex auch katalytisch aktiv für die akzeptorfreie Dehydrierung von Alkoholen zu Estern und einige andere Reaktionen.
________________________________________________________________
Editorial publications
1.) "Nanocatalysis in Ionic Liquids", Wiley-VCH (Weinheim), Ed. Martin H. G. Prechtl, ISBN: 978-3-527-33910-5, on 12/2016. Wiley: http://eu.wiley.com/WileyCDA/WileyTitle/productCd-3527339108.html Electronic Version: http://onlinelibrary.wiley.com/book/10.1002/9783527693283
Direct purchase here at www.gartenstadtbuecher.de :
Hardcover version of "Nanocatalysis in Ionic Liquids" (external Link)
PDF version of "Nanocatalysis in Ionic Liquids" (external Link)
ePub/eBook version of "Nanocatalysis in Ionic Liquids" (external Link)
2.) "Nanoscale Catalysts as Tools for Synthesis", Special Issue, Guest Editor: Martin H. G. Prechtl, Current Organic Chemistry 2013, 17, 4, 325-445. Editorial DOI: http://dx.doi.org/10.2174/1385272811317040002 Issue: http://benthamscience.com/journal/contents.php?journalID=coc&issueID=107628
3.) "Small molecules for hydrogen storage", Special Issue, Coordinating Editor: Martin H. G. Prechtl, Recyclable Catalysis 2015, 2, 1. Editorial http://degruyteropen.com/tirecat/ http://degruyteropen.com/people/mhgprechtl/ Special Issue: http://www.degruyter.com/view/j/recat.2015.2.issue-1/issue-files/recat.2015.2.issue-1.xml